Background - We reported the efficacy and safety of using a combination of ibrutinib plus rituximab (IR) induction followed by short course (4 cycles) of R-HCVAD/MTX-ara-C as consolidation in previously untreated young (age ≤ 65 years)patients (pts) with mantle cell lymphoma (MCL), Wang M et al Lancet Oncology 2022. Here we report the long term follow up of this study.
Methods - We enrolled 131 previously untreated pts in this single institution, single arm, phase II clinical trial - NCT02427620. Pts received IR induction (part-A), until they achieved complete remission (CR) for up to a maximum of 12 cycles, followed by a maximum of 4 cycles of R-HCVAD/R-MTX-ara-C (part-B) as consolidation. The primary objective was to assess overall response rate (ORR), [defined as either a partial response (PR) or a complete response (CR)] after part A. Adverse events were coded as per CTCAE version 4. High risk pts received ibrutinib for 1 year and rituximab once every 2 months for 24 months after completion of part B.
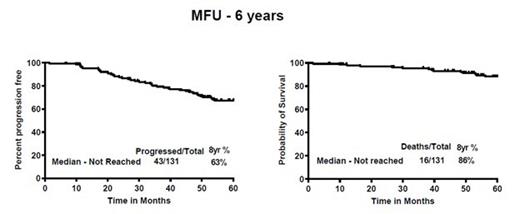
Results - Among the 131 pts, the median age was 56 yrs (range - 35-65). High Ki-67 (≥30%) in 58/117 (49.5%) pts, 10 pts (8%) had high risk simplified MIPI score, 15 pts (11%) had aggressive MCL (blastoid/pleomorphic) and 114 pts (87%) had initial bone marrow involvement. Fifty percent of the patients had Ki-67 (≥30%). ORR in part-A was 98% (87% CR). After completion of part A and part B, ORR was 90% (89% CR). After a median follow up of 71 months, 43 patients progressed/died. The median progression free survival (PFS) and overall survival (OS) were not reached. Overall, 30 pts (23%) relapsed after treatment, including 5 who transformed to aggressive MCL. PFS among pts with high and low Ki-67% was 42 months vs not reached (P=0.01) while no difference was observed in OS. PFS was significantly shorter in pts with aggressive histology (p=0.001) but not the OS. PFS was similar in pts who received maintenance vs no maintenance therapy. Overall, 8 pts died (4 with progression, 2 due to disease transformation, one on study due to multiple complications including splenic hematoma, cardio-pulmonary arrest and progression and the last one expired outside and came off study due to encephalitis). 50 pts came off study for various reasons [30 disease progression (including 5 transformation), 10 pt choice, 8 intolerance, 1 second cancer and 1 lost to follow up]. Long term, grade 3-4 toxicities on part A were 6% myelosuppression and 10% each with fatigue, myalgia and rashes and 2% mucositis. Maintenance therapy did not show high toxicities.
Conclusions -Long term follow up of chemo-free induction with IR induced durable and deep responses in young MCL pts in the frontline setting. Short course R-HCVAD chemotherapy minimized toxicities and consolidated responses. IR maintenance improved survival outcomes. This combined modality treatment approach may significantly improve young MCL pts outcomes across all risk groups.
Disclosures
Jain:AstraZeneca: Consultancy, Honoraria. Lee:Guidepoint: Honoraria; Celgene: Research Funding; Cancer Experts: Honoraria; Deloitte: Honoraria; Bristol-Myers Squibb: Research Funding; Seagen Inc.: Research Funding; Century Therapeutics: Consultancy; Aptitude Health: Honoraria; Oncternal Therapeutics: Research Funding; Curio Sciences: Honoraria; Janssen: Honoraria; Takeda: Research Funding; Pharmacyclics: Research Funding; Olson Research: Honoraria; Korean Society of Cardiology: Honoraria. Westin:Calithera: Research Funding; SeaGen: Consultancy; Abbvie: Consultancy; MonteRosa: Consultancy; Nurix: Consultancy; Novartis: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; BMS: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding; Kymera: Research Funding. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Regeneron: Honoraria; AstraZeneca: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; AbbVie: Honoraria; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria. Iyer:Yingli: Consultancy, Research Funding; CRISPR: Consultancy, Research Funding; Innate: Research Funding; Acrotech: Consultancy, Research Funding; Legend: Research Funding; Astra Zeneca: Research Funding; Ono: Research Funding; Pfizer: Research Funding; Salarius: Consultancy; Drenbio: Research Funding; Merck: Research Funding; American Society of Transplant and Cellular Therapy: Speakers Bureau; CuraBio: Speakers Bureau; American Society of Hematology: Speakers Bureau; Seagen: Consultancy, Research Funding. Vega:Geron: Research Funding; Allogene: Research Funding. Flowers:Eastern Cooperative Oncology Group: Research Funding; Genmab: Consultancy; Iovance: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Burroghs Wellcome Fund: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; National Cancer Institute: Research Funding; Allogene: Research Funding; Ziopharm: Research Funding; Xencor: Research Funding; Takeda: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Adaptimmune: Research Funding; Amgen: Research Funding; Jannsen Pharmaceuticals: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Karyopharm: Consultancy; 4D: Research Funding; Acerta: Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech Roche: Consultancy, Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Spectrum: Consultancy; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; Abbvie: Consultancy, Research Funding; TG Therapeutics: Research Funding; Gilead: Consultancy, Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Wang:Miltenyi Biomedicine: Consultancy; Genmab: Honoraria, Research Funding; i3Health: Honoraria; Merck: Consultancy, Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; MJH Life Sciences: Honoraria; Moffit Cancer Center: Honoraria; MD Education: Honoraria; DTRM Biopharma (Cayman) Limited: Consultancy; NIH: Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Nurix: Honoraria; IDEOlogy Health: Honoraria; Dava Oncology: Honoraria, Other: Travel; Bantam Pharmaceutical: Honoraria; Pepromene Bio: Consultancy; Pharmacyclics: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Milken Institute: Consultancy; Oncternal: Consultancy, Research Funding; Parexel: Consultancy; Eastern Virginia Medical School: Honoraria; Leukemia & Lymphoma Society: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Other: Travel, Research Funding; Genentech: Consultancy; InnoCare: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; CAHON: Honoraria; Juno Therapeutics: Research Funding; Genentech: Research Funding; Celgene: Other: Travel, Research Funding; WebMD: Honoraria; Studio ER Congressi: Honoraria; Scripps: Honoraria; Practice Point Communications (PPC): Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; Oncology Specialty Group: Honoraria; OncLive: Honoraria; Deciphera: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Be Biopharma: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Loxo Oncology: Research Funding; Molecular Templates: Research Funding; Vincerx: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal